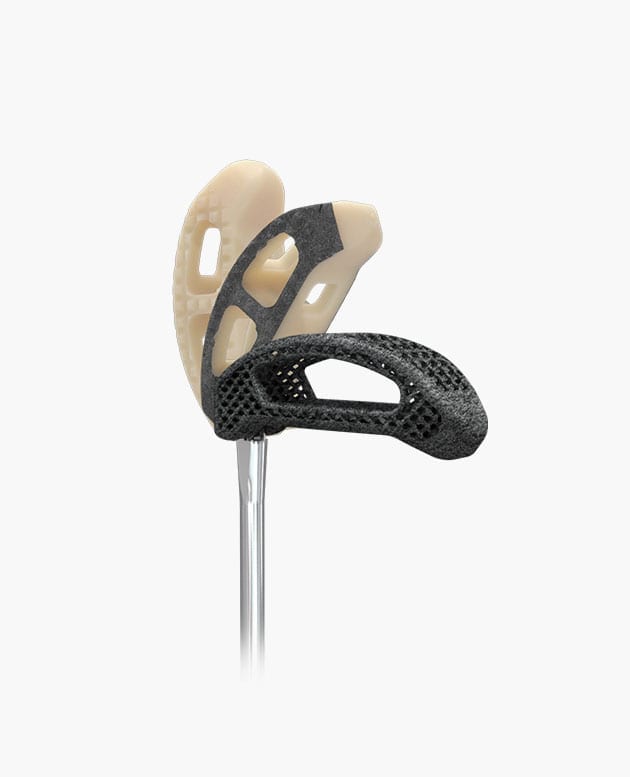
Idys®-TLIF
Pivoting TLIF Cage
Smart pivoting system
Controlled pivoting system, optimal implant positioning, reliable and repeatable surgical technique.
Bullet-shaped nose
Self-distracting bullet nose facilitates insertion especially into collapsed disc spaces.
Anatomical shape
Anatomical shape conforms to the patient’s vertebral endplates to provide enhanced stability.
| Registration US Clearance: Yes | |
| Registration US Clearance: Yes | |
| Registration US Clearance: Yes |